UK may become one of the fastest countries to approve jabs for Covid variants
The UK could become one of the fastest countries in the world to approve new Covid-19 vaccines to tackle variants.
Health Secretary Matt Hancock said the Medicines and Healthcare products Regulatory Agency (MHRA) will oversee a fast-track approach to approving new jabs, after studies suggested variants may make vaccines less effective.
During a visit to the Glasgow lighthouse lab, Mr Hancock said: “We will have a fast-track approach to safely approving future vaccines that work against a variant of Covid-19.
“The vaccine programme has clearly been a huge UK success story, and part of the reason that we have been able to develop the vaccines so far, so quickly, is because of the MHRA’s rigorous yet flexible approach, which has been based entirely on looking as quickly as possible at the safety and efficacy of vaccines.
We've published new guidance on the information we would need to approve any modifications to authorised COVID-19 vaccines, should virus mutations make them less effective at preventing the disease.
Find out more: https://t.co/GV7DwTKAje pic.twitter.com/FTw9gDmt0s
— MHRAgovuk (@MHRAgovuk) March 4, 2021
“I’m delighted that they’re taking that same principled approach to the approval process for vaccines that may work against variants.”
Scientists have become concerned about several variants, including one first identified in the Brazilian city of Manaus.
A study this week suggested that between 25% and 61% of people in the city who had previously had Covid were susceptible to reinfection with the worrying P1 variant found there.
Six cases of P1 have been found in the UK to date – three in England and three in Scotland.
Vaccine manufacturers including Pfizer and AstraZeneca are already working on new jabs to tackle variants in case they are needed.
MHRA chief executive Dr June Raine said the new approval process is “agile”, and the green light for new jabs could be given in just a few weeks after all data has been submitted on their effectiveness.
She insisted no corners would be cut on safety, and the framework already exists for when annual flu jabs are tweaked.
She said: “Has this approach been done before? This approach that we’re putting out today for the Covid-19 vaccines is based on the tried and tested regulatory process which is used every year for seasonal flu jabs.”
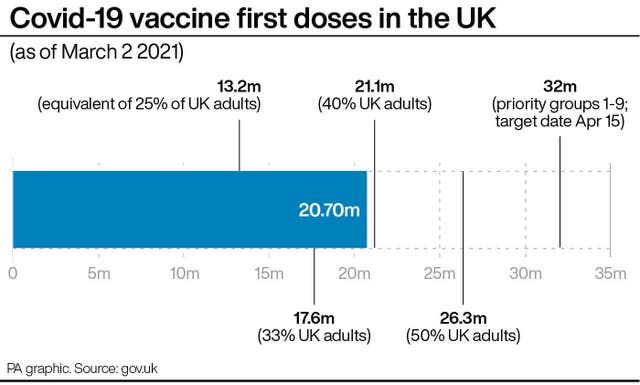
She said that by carrying out a “real-time” rolling review of evidence, as was done for the original Covid-19 vaccines, the MHRA’s approval would come “in a very efficient manner, as short as a couple of weeks”.
Asked about whether the UK would offer quicker approval than some other countries, she said: “You will be aware that there are other major jurisdictions, the FDA (in the US) and the European Medicines Agency, and this guideline we feel is more flexible and more capable of moving in an agile way, depending on the format of the vaccine, and the data we already hold.
“You’ll be aware that the data we hold in the UK, having been agile initially, is a very substantial set of data.”
The MHRA has joined forces with regulators in Australia, Canada, Singapore and Switzerland as part of the ACCESS Consortium, which has issued new guidance on rapid approval.
Dr Raine said the focus at the MHRA “is to make sure that innovation reaches patients in the shortest time”, with the “hallmark” of the latest guidance being “scientific flexibility”.
She added: “Our flexible guideline is what we believe will enable us to adapt very quickly to changing situations which, as we know, can change very fast.”
The MHRA has said researchers can measure protection by looking at antibodies in the blood after vaccination, reducing the need to wait and see whether people in a clinical trial become infected with the virus.
This will significantly reduce the time it takes for the modified vaccine to be ready, with new trials involving perhaps just a few hundred people.
Dr Christian Schneider, chief scientific officer at the MHRA, said: “The public should be confident that no vaccine would be approved unless the expected high standards of safety, quality and effectiveness are met.”
In other developments:
– An independent vaccine panel in Germany has formally approved the AstraZeneca vaccine for people aged 65 and over. The country previously restricted it to under-65s, citing insufficient data of its effects on older people.
– More than four in 10 over-80s who received a coronavirus vaccine during the current lockdown appear to have since broken the rules by meeting up indoors with someone not in their household, support bubble or a carer, new figures from the Office for National Statistics suggest.
– Prime Minister Boris Johnson said he is “doing all I can to lose weight” as he announced funding to help the public get “fitter and healthier”.
– Chancellor Rishi Sunak said it is “too early” to say whether the Government can support rolling out vaccine passports while a review is under way.
Meanwhile, Mr Hancock promoted the idea of a “great British summer” during his Glasgow visit.
“I very much hope that as we are able to lift restrictions, then we are all able to travel across the UK,” he said.
“I’m confident because of the vaccine we will be able to make that progress and then be able to, all of us, to travel freely wherever we are within these islands
“One of the factors that we have to be vigilant about in that road map is the emergence of new variants, in case the current vaccines are not as effective.
“I’ve said before that I’m optimistic for a great British summer and I’m now more optimistic about having a great British summer than I have been at any time, thanks to the speed and the effectiveness of the vaccine rollout.
“By great British summer, I absolutely mean people being able to enjoy travel across the whole of the UK.”
