There’s a problem that caused one batch of insulin injection pens to be recalled
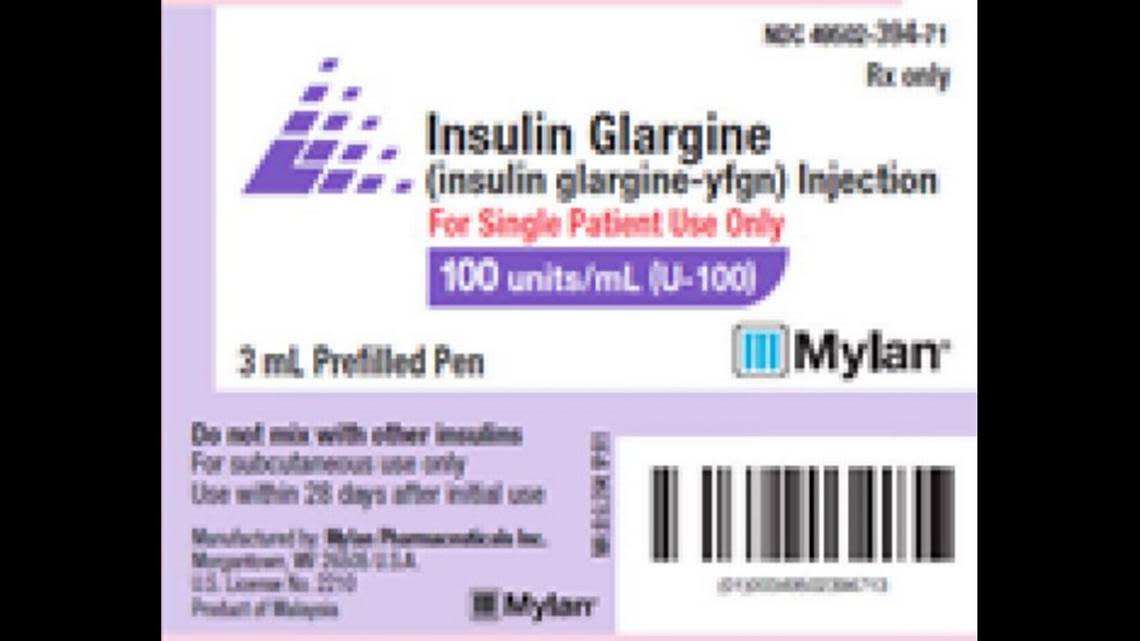
Mylan Pharmaceuticals recalled one batch of Insulin Glargine Injection, 100 units/mL (U-100), 3 mL prefilled pens, which are sold in cartons of five. Some pens might be missing their labels.
In explaining the problem that could cause, the Mylan-written, FDA-posted recall notice says: “For patients receiving treatment with more than one type of insulin (e.g., both short and long-acting insulin), a missing label on Insulin Glargine pens could lead to a mix-up of products/strengths, which may result in less optimal glycemic control (either high or low blood sugar) which could result in serious complications.”
Mylan also makes the distinction that this isn’t the Semglee brand pen, but unbranded Insulin Glargine-yfgn pens.
This covers batch No. BF21002895, expiration August 2023.
Wholesalers should quarantine what they have and email a list of what they’ve already distributed in a Microsoft Excel file to mylan8775@sedgwick.com.
Consumers who have an unlabled pen should contact Sedgwick, which is handling this recall for Mylan, at 877-643-8438 for a packet to return the pen.
If you have questions regarding this recall, email at customer.service@viatris.com or call Viatris at 800-796-9526, Monday through Friday, 8 a.m. to 5 p.m., Eastern time.
If this or any drug causes a problem, after notifying a medical professional, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088. Then, notify the manufacturer.
