Pig heart transplants in humans show signs of success
New research in which doctors transplanted genetically modified pig hearts into people who were clinically dead could pave the way for human trials and a future with more organ transplants that can prolong lives.
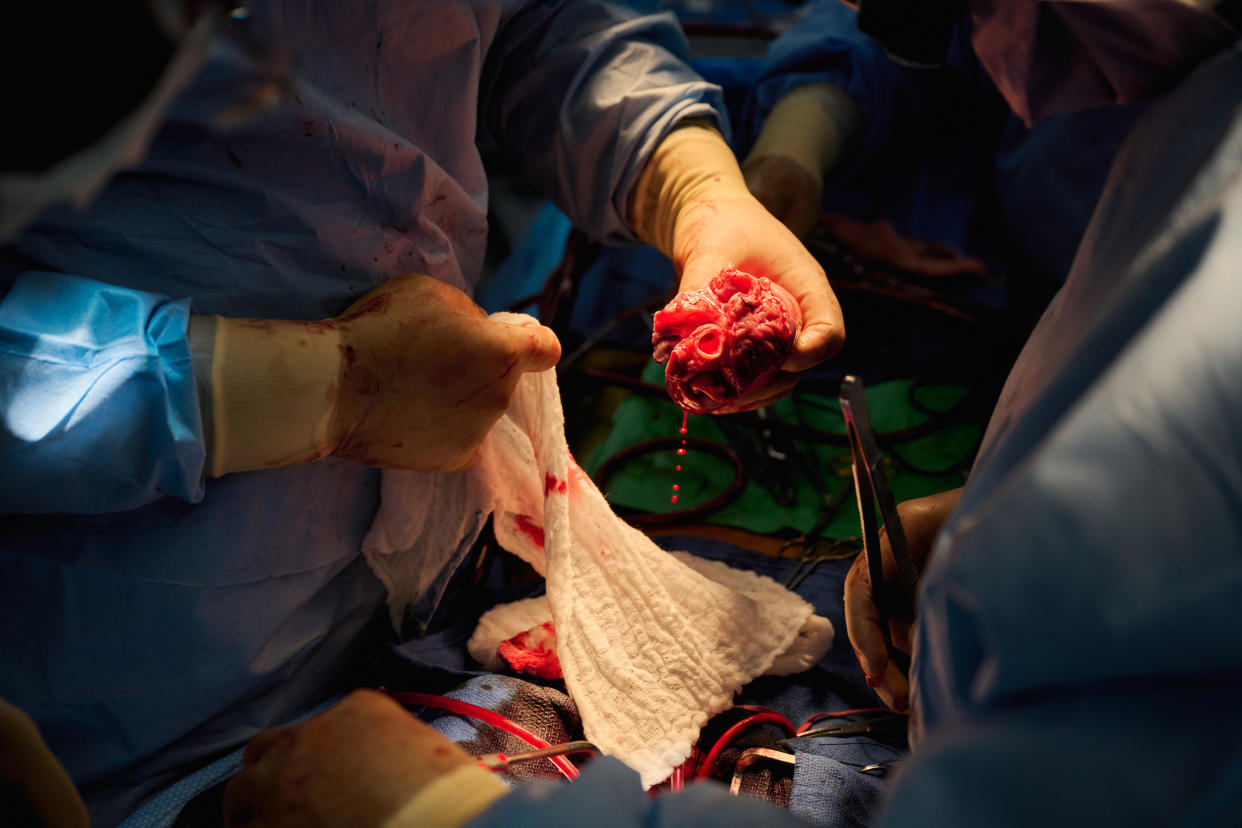
In the past month, researchers at NYU Langone Health transplanted pig hearts into two people who had recently suffered catastrophic heart failure and were left brain dead but remained on life support.
In both cases, the new hearts beat strongly and were not immediately rejected by the host bodies. The hearts continued to function well until the conclusion of the three-day experiment, doctors said.
“The heart was literally banging away. It was contracting completely normally,” Dr. Nader Moazami, a surgeon who was part of the transplant team, said of the moments after the heart restarted. “We learned a tremendous amount.”
Doctors at the University of Maryland last year implanted a pig heart into a living patient, but it failed after 49 days. The NYU research in subjects considered deceased is different because it allows researchers to rigorously test, refine treatments and collect detailed data without fear that experimentation will take a patient’s life.
The doctors hope that their research model — of testing pig organs in clinics with deceased patients — can help prepare the medical community for clinical trials and reduce the chances that living patients’ immune systems will turn on new organs. Nationwide, fewer organs are available for transplant than are needed by patients; pig organs could expand access to transplants and allow doctors to broaden who is eligible for such procedures.
“It’s all about going into the first living human trials with as much data as we can possibly have and make it as safe as possible — and effective,” said Dr. Robert Montgomery, the director of the NYU Langone Transplant Institute.
Doctors have long pursued new ways to meet the nation’s need for transplant organs.
In 2021, more than 116,000 people in the United States were accepted to waiting lists for an organ transplant, according to the national Organ Procurement and Transplantation Network.
The same year, 6,166 people died while waiting for their number to be called.
“There just aren’t enough organs out there to meet the needs of people with organ failure,” said Dr. Megan Sykes, the director of the Columbia Center for Translational Immunology, who was not part of the NYU heart project.
Organ scarcity ebbs and flows, but the sources of organs today reflects some of society’s woes — with victims of car crashes, opioid overdoses and gunshot wounds making up a large portion of donors, according to Dr. Maryjane Farr, a professor of medicine and the heart failure section chief at UT Southwestern Medical Center in Dallas who was not involved in the NYU research.
The current system doesn’t offer much for some patients, including young children with heart issues, because their peers are largely staying out of harms’ way.
“It’s almost impossible for a 1-year-old or 2-year-old with end stage congenital heart disease to get a donor,” Farr said. “There are populations for which xenotransplants are going to be the only way," she said, using the term for the transplantation of organs from one species to another.
Over decades, doctors have inched closer to figuring out how to make animal organs suitable for people.
Researchers have focused recent efforts on pigs because their organs are similar in size to humans, and they have large litters and are easy to modify genetically, Montgomery said. Pigs are slaughtered by the millions for food in the U.S., which makes using their organs to sustain human life more palatable to the public than using primates, he added.
Before they are transplanted, pig hearts require genetic modification to reduce the risk of rejection and to ensure proper function. Researchers “knock out” — or silence — particular genes to prevent human antibodies from attacking the new organ when it is connected, Montgomery said. The researchers also prevent the expression of genes that would allow the heart to grow larger once inside the person receiving the transplant and exposed to human growth hormone. Researchers also “knock in” certain genes to perform some important human biological processes.
Without genetic modification, a patients’ heart rate would skyrocket and the organ would “turn black within a minute or two,” Montgomery said.
Transplant experiments from pigs to laboratory primates have been a key source of progress, Montgomery said. But primates are hard to care for, are prone to infection, and their bodies don’t always react the same as humans.
“In the last few years, we’ve seen survivals of pig hearts in nonhuman primates going six to nine months. That’s huge and that’s really a big advance,” Sykes said. “It’s come to a point where a lot of people feel it’s time to start studies in people.”
Montgomery and other doctors last year began to transplant pig kidneys into human subjects who were brain dead and remained on life support.
And while the first pig heart transplant in a living patient ended in the patient's death in Maryland nearly two months later, his outcome was complicated by a pig virus later found in the transplanted heart. Why the 57-year-old man’s heart ultimately failed remains in question.
For the NYU experiments, researchers worked for several years to develop protocols and to establish an independent board that would evaluate the ethical considerations of transplanting a pig heart into a person who had recently died.
In mid-June, researchers secured consent to operate on the body of a 72-year-old man who had a heart attack while driving and was brain dead but remained on life support.
A team of doctors flew to Virginia, removed the heart from a pig, put it on ice and flushed it with preservation fluid and then flew it back to Moazami.
The heart was smaller than expected and Moazami had to improvise to connect some of the vessels that needed to be connected.
Other than that, “we performed the operation just as if we were going to do a clinical human to human transplant,” Moazami said. The entire process — including transportation — took about four hours and 20 minutes.
The hospital converted the operating room into an intensive care unit, where the subject was observed for three days before life support was removed. Doctors were able to take regular biopsies and blood tests throughout the process, which will give them data to study later.
Last week, doctors performed the same process again, operating on a deceased woman in her 60s. They reduced the combined time of transportation and operating with the pig heart by about 50 minutes.
Doctors learned from the Maryland case and implemented more sensitive testing for the pig virus that might have complicated the patient’s recovery there. The virus was not detected in either NYU subject.
Montgomery hopes that by combining short-term studies of transplants in the deceased, monthslong studies of pig heart transplants in primates and one-off studies like the one in Maryland, researchers can make a strong case to the federal Food and Drug Administration to greenlight clinical trials.
“Obviously, actually having these organs in humans has created an incredible amount of forward inertia and excitement in the field and accelerated things,” Montgomery said. “It still is a bit of a moving target. We think it’s going to be in the next year or two.”
The partner of the 72-year-old man whose body was used for the first NYU heart xenotransplant hopes his legacy will now include helping to save children who might otherwise be waiting on a functional heart.

In mid-June, Alice Michael was talking on the phone with her partner of 33 years, Lawrence “Larry” Kelly, when he had a heart attack.
By the time she arrived at the hospital, doctors told her that her partner of 33 years, the kindhearted man who loved hunting, finding treasures with his metal detector and helping disabled veterans access their benefits, had been left without brain function.
A Vietnam veteran of the U.S. Navy, Kelly had his first heart attack in 1993 and weathered two open-heart surgeries. Troubles with his heart had weighed on Kelly for much of his adult life.
Michael said she didn’t hesitate when she received a call from NYU about using Kelly’s body in research.
“I didn’t even have to think twice,” Michael said. “He was a hero his whole life and he went out a hero. That comforts me a lot knowing that he helped other people.”
