Oak Ridge National Laboratory credited with discovery of 3 elements in the periodic table
One of the proud achievements of Oak Ridge National Laboratory is the discovery of three elements either by its own staff or in collaboration with other institutions in the United States and Russia. Carolyn Krause provides a history of these discoveries in a three-part series based mostly on a talk last fall by Jim Roberto, who held many leadership positions at ORNL, to a class of the Oak Ridge Institute for Continued Learning (ORICL).
***
Oak Ridge National Laboratory is credited with the discovery of three elements. All of them were originally “missing” from a nearly completed row in the periodic table – the organized array of all the chemical elements in order of increasing atomic number. That’s the total number of protons in an atomic nucleus of each element.
The three elements are promethium (element 61), moscovium (element 115) and tennessine (element 117), which is named after Tennessee. Moscovium is named after Moscow – that will be explained in the second part of this series. In addition, nine elements have been discovered using ORNL isotopes.

An ORICL class recently learned these facts while listening to a fascinating history of element discovery involving ORNL presented by Jim Roberto. While at ORNL he served as director of the Solid State Division, associate laboratory director for physical sciences, deputy director for science and technology and associate laboratory director for partnerships.
He started his talk by discussing the first modern periodic table produced in 1869 by Dmitri Ivanich Mendeleev (1834-1907), a Russian chemist who developed the periodic classification of the elements according to each one’s atomic mass (atomic weight, based on the total number of protons and neutrons in each element’s atomic nucleus).
“The mass of each of the elements didn’t put them in the right place in the periodic table according to the pattern of their chemical properties,” Roberto said. “The reason is that the determination of the periodic law is not the mass. It’s the atomic number - the number of protons each element has.”
The atomic number for each known element was determined in 1913 by Henry Gwyn Jeffreys Moseley (1887-1915), a brilliant young British scientist who was killed fighting in World War I. He collected the X-Ray spectra of a variety of elements and found that the frequency of X-Ray radiation has a precise mathematical relationship to each element’s atomic number.
This relationship, in which X-Ray frequency is proportional to atomic number, is called Moseley’s Law. According to the law, elements arranged in the order of their atomic numbers show a periodic variation in atomic structure and most of their properties. Moseley discovered that element 61 was missing, a gap in the periodic law where this element should appear. That’s where Oak Ridge comes into the picture.
By 1939, 91 elements were known to exist, including uranium, which has 92 protons in each nucleus and, at the time, the highest atomic number in the periodic table. But element 61 was still not known to exist.
During the Manhattan Project, workers in Oak Ridge built the Graphite Reactor in 1943, then called the X-10 pilot plant at Clinton Laboratories. It was used to demonstrate that plutonium could be made from uranium fuel slugs bathed in neutrons at the reactor. Small amounts of plutonium from the reactor were separated from spent uranium slugs and sent by train to Los Alamos National Laboratory.
There it was determined that a new weapon design was needed to make an atomic bomb that would use as its fuel the larger amounts of plutonium expected to come from three big reactors in Hanford, Washington. A detonation to demonstrate that design works was the most dramatic scene in last year’s blockbuster “Oppenheimer” movie.
Element 61 was missing, Roberto said, “because it's radioactive. Shortly after Earth was born as a planet, element 61 mostly decayed away and could not be found. But element 61 was discovered in Oak Ridge during the Manhattan Project at the Clinton Laboratories’ Graphite Reactor.”
Element 61 was first produced and characterized at the reactor in 1945 by Charles Coryell, Jacob A. Marinsky and Lawrence E. Glendenin. The team kept their discovery secret for security reasons because World War II was underway. In 1947, they announced they had found a new decay product of uranium fission in the spent slugs from the Graphite Reactor. It turned out to be the missing element 61.
Grace Mary Coryell, the wife of Coryell, director of the lab’s chemistry division, suggested that the element be named “prometheum,” after Prometheus, a figure in Greek mythology who is considered a champion of humankind. He defied the gods by stealing fire from Mount Olympus and bringing it to humans. The name was accepted by the International Union of Pure and Applied Chemistry, which changed “prometheum” to “promethium” to comply with its spelling standards for elements in the periodic table.
Roberto noted that for national security reasons, a copy of an updated periodic table was displayed in 1945 in a secure place at the Y-12 electromagnetic plant for workers involved in isotope separation, a long-time Y-12 mission starting with uranium isotopes during the war. The periodic table included promethium and three newly discovered elements heavier than uranium - americium, curium and plutonium.
Promethium, which has the symbol Pm, was the last rare-earth element of the lanthanide series to be discovered. According to Wikipedia, most promethium-147 is utilized for research. It can be used as a beta radiation source in luminous paint; in thickness-measurement devices; in nuclear batteries for guided missiles, watches, pacemakers and radios, as well as a light source for signals.
Because natural promethium is scarce (it can be made by rare alpha-particle decays of natural europium-151), Pm-147 is typically synthesized by bombarding uranium-235 in enriched uranium with thermal neutrons in a reactor. The first sample of the highly radioactive metal made as a fission product was produced in 1963.
Promethium is only one of the many radioisotopes that ORNL produced at the Graphite Reactor and other reactors. The first one shipped went to a St. Louis hospital in 1946 for cancer research. In the 1960s more than 100,000 radioisotope shipments were made annually for use in research, medicine and industry. Because of the growing demand for certain isotopes and the need to reduce U.S. dependence on foreign supplies, at ORNL the Stable Isotope Production Facility is under construction, and the Radioisotope Processing Facility has been designed. Its opening behind the High Flux Isotope Reactor (HFIR) is planned for the 2030s.

Roberto then talked about the first efforts to create transuranium elements, which are not found in nature. In 1934, Enrico Fermi attempted to create heavier elements by irradiating uranium with neutrons in his lab in Italy. Because he identified a new type of radioactivity from products with atomic chemistry different from that of uranium and similar elements, he claimed in his published paper that he had found evidence of a new transuranic element, findings believed to be correct for several years.
Ida Noddack, a German chemist and co-discoverer of rhenium (element 75), quickly published a paper questioning Fermi’s conclusion. She pointed out that he had failed to chemically eliminate all elements lighter than uranium in his proofs and to consider that “it is conceivable that the nucleus breaks up into several large fragments, which would of course be isotopes of known elements but would not be neighbors of the irradiated element.”
Her findings were dismissed as untrue in the 1930s, but her paper, according to Wikipedia, “is considered historically significant today” because her conclusion “presaged what would become known” in 1939 as nuclear fission, first described and named in a scientific paper by another woman scientist, Lise Meitner (in collaboration with her nephew and two German experimental scientists, who won the Nobel Prize in Chemistry in 1944). As Roberto put it, “Fermi missed the discovery of nuclear fission” that the German scientists were acclaimed for making.
However, on Dec. 2, 1942, at the Chicago Pile-1, Fermi, then a Nobel laureate, led the team that advanced the understanding of how to exploit nuclear fission by demonstrating the first human-created, self-sustaining nuclear chain reaction. This discovery made possible the 14 reactors built at ORNL.
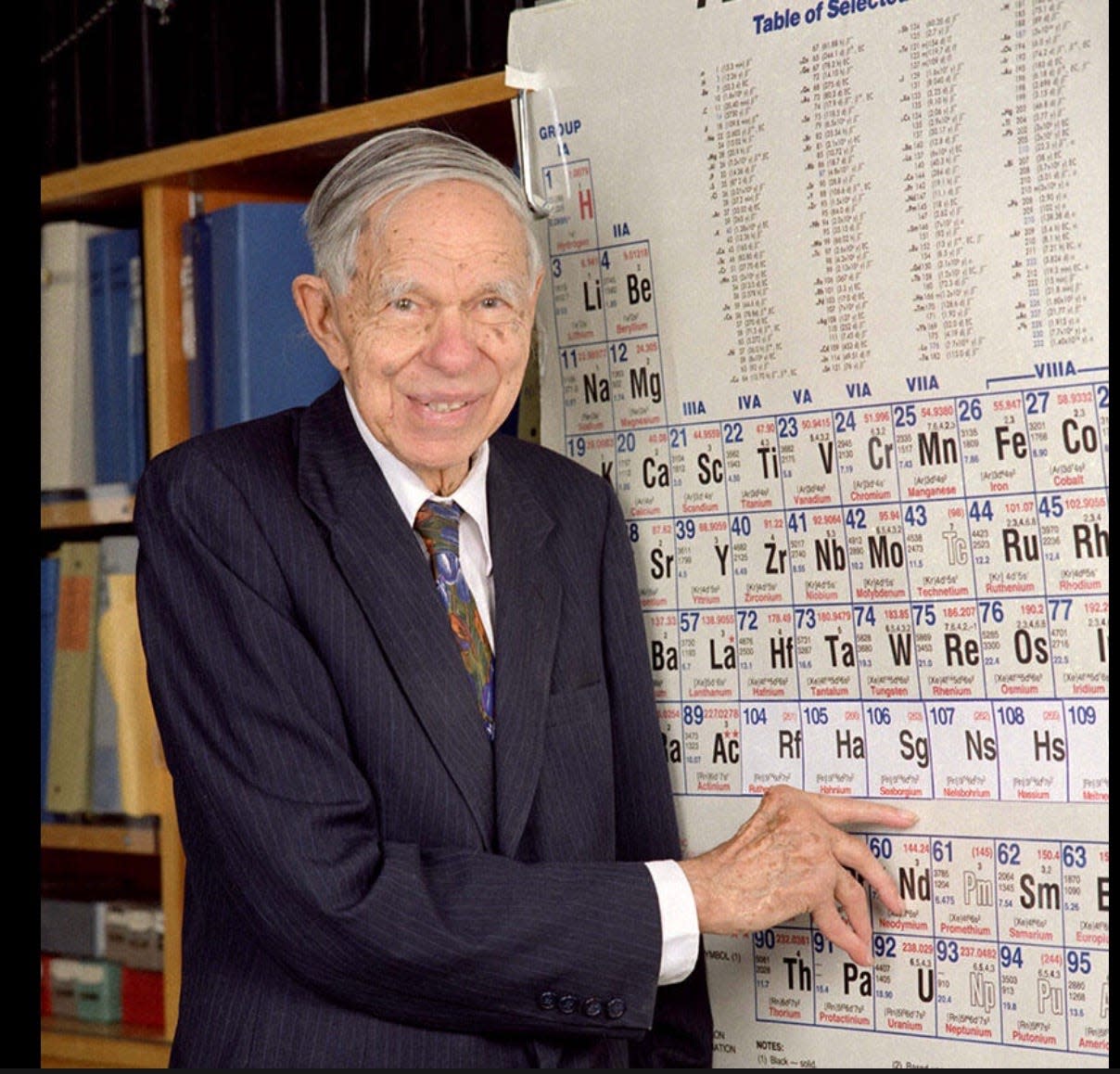
A Nobel laureate who spearheaded the first discoveries of transuranium elements was Glenn Seaborg. In December 1940, his team working at the 60-inch cyclotron at the Lawrence Radiation Laboratory at the University of California at Berkeley produced man-made plutonium (element 93). They created the element by bombarding a uranium target with a beam of deuterons (hydrogen nuclei consisting of a proton and a neutron).
Seaborg and Edwin McMillan jointly won the Nobel Prize in Chemistry in 1951 “for their discoveries in the chemistry of the transuranium elements.” However, there is strong evidence, as pointed out in past "Historically Speaking" columns, that Seaborg’s close friend Stanley Thompson made improvements in chemical separation techniques and radiation detectors that enabled him and his team to synthesize and identify five transuranium elements that helped Seaborg win a Nobel prize: berkelium (97 protons) and californium (98) in 1949-50 and einsteinium (99), fermium (100) and mendelevium (101) in 1952 and 1953.
Seaborg proposed a new row for the periodic table called the actinides to accommodate these newly discovered elements, including elements 102 and 103 (nobelium and lawrencium), Roberto said. Seaborg also envisioned the existence of superheavy elements with atomic numbers greater than 103 that would continue row 7 of the periodic table.
Seaborg knew that the only way to get a high enough yield of transuranic elements to determine their chemical properties would be in a high-flux nuclear reactor in which targets are struck by an extremely high number of neutrons per unit area per second. So, Seaborg wrote a letter to Lewis Strauss (1951-58), then chairman of the Atomic Energy Commission and the villain in the “Oppenheimer” movie.
Many ORNL staff considered Strauss the hero of the High Flux Isotope Reactor, the reactor that Seaborg, who later became AEC chairman in 1961, asked to be built at ORNL. The reason: Strauss received the HFIR proposal from Oak Ridge and he strongly advocated at a Nov. 24, 1958, hearing that a high-flux reactor be built.
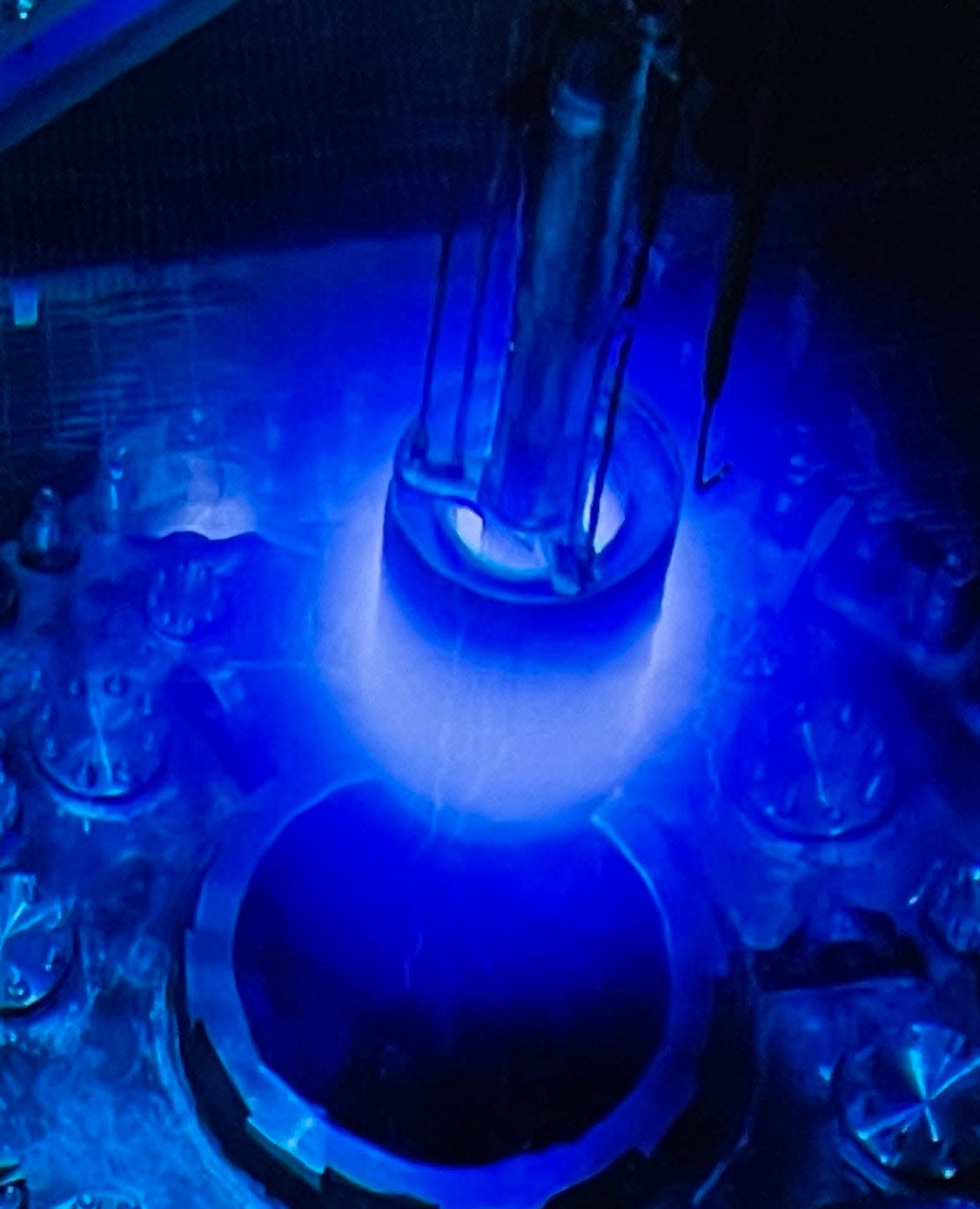
HFIR started operation at ORNL in 1966. HFIR has created gram quantities of californium and lesser amounts of berkelium and einsteinium, enabling the study of the three transuranic elements and their unique chemistry. And 53 years after Seaborg wrote his letter to Strauss, on Apr. 5, 2010, tennessine (element 117, named after Tennessee) was discovered in Russia using berkelium produced in Oak Ridge at HFIR!
***
Thank you, Carolyn. Next week read Part 2 of the three-part series on the discovery of elements by ORNL.
D. Ray Smith is the city of Oak Ridge historian. His "Historically Speaking" column appears each week in The Oak Ridger.


This article originally appeared on Oakridger: ORNL credited with discovery of 3 elements in the periodic table
