FDA to approve new omicron COVID boosters
The Food and Drug Administration is set to approve new boosters that specifically target the omicron mutation of COVID-19.
Both the Moderna and Pfizer vaccines have been updated to protect against the highly contagious variants as well as the first strain.
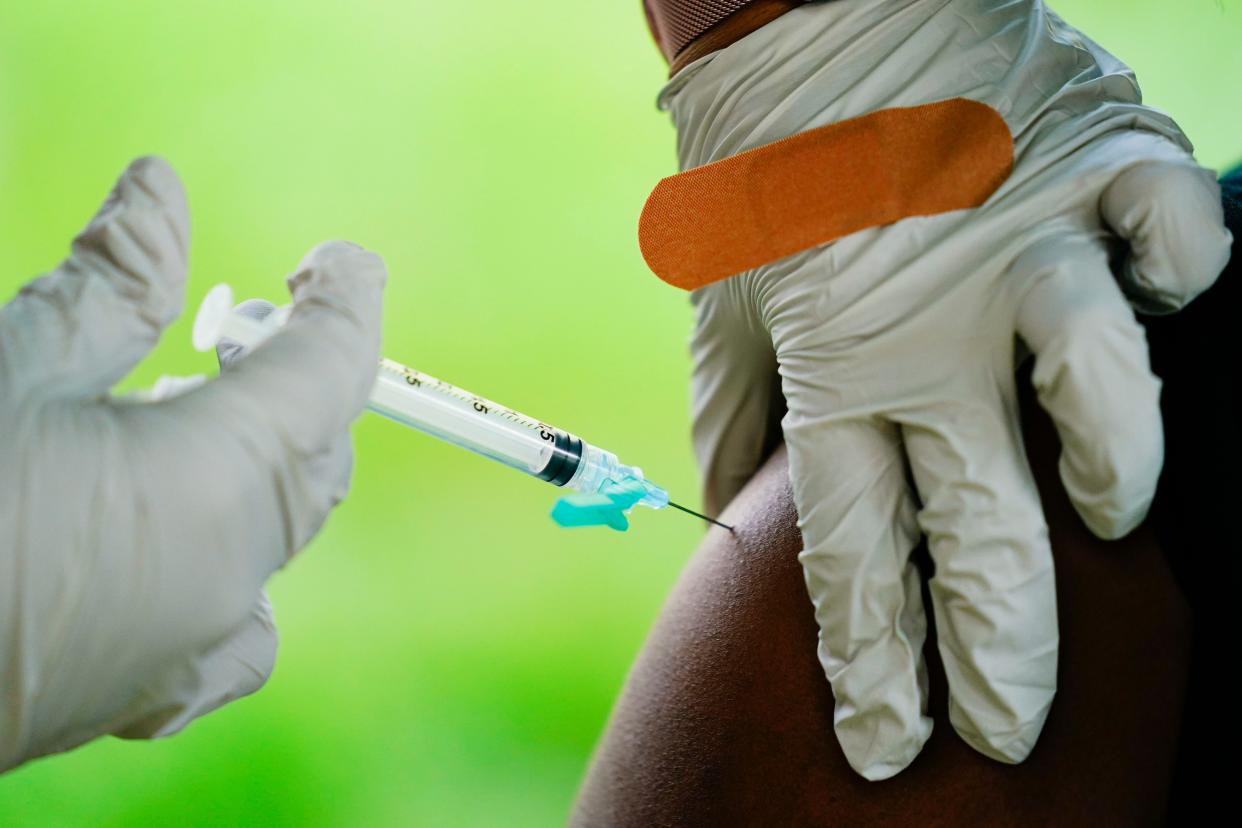
A health worker administers a dose of a Pfizer COVID-19 vaccine. (Matt Rourke/)
The omicron variants have long been the dominant strains, leading to reinfections and an increase in vaccinated people contracting the virus.
“This is a really important moment in this pandemic,” Dr. Ashish Jha, the White House COVID-19 Response Coordinator, told NPR. “This is the first major upgrade of the vaccines — first major change in the vaccines — in the last two and a half years.”
The White House has already purchased more than 170 million doses of the new boosters and plans to roll out the shots as soon as possible to hopefully head off a surge in cases in the fall and winter similar to the surge the U.S. witnessed near the end of 2021.
The U.S. Centers for Disease Control and Prevention will give the final authorization after the FDA’s approval is official.
