Nasal vaccine for treating, preventing Alzheimer’s disease to be tested in Boston trial
Researchers working to combat Alzheimer’s disease will soon kick off a “landmark” clinical trial, in which they’ll administer a nasal vaccine to participants at a Boston hospital.
Each of the study’s 16 participants, aged between 60 to 85 years old, was diagnosed with Alzheimer’s and is dealing with the early stages of the disease, said the Brigham and Women’s Hospital, where the trial is taking place.
Experts hope the trial will show the vaccine can “prevent and slow the progression” of Alzheimer’s, an illness that causes memory loss.
“The immune system plays a very important role in all neurologic diseases,” said Howard L. Weiner, the Ann Romney Center for Neurologic Diseases’ co-director.
“And it’s exciting that after 20 years of preclinical work, we can finally take a key step forward toward clinical translation and conduct this landmark first human trial.”
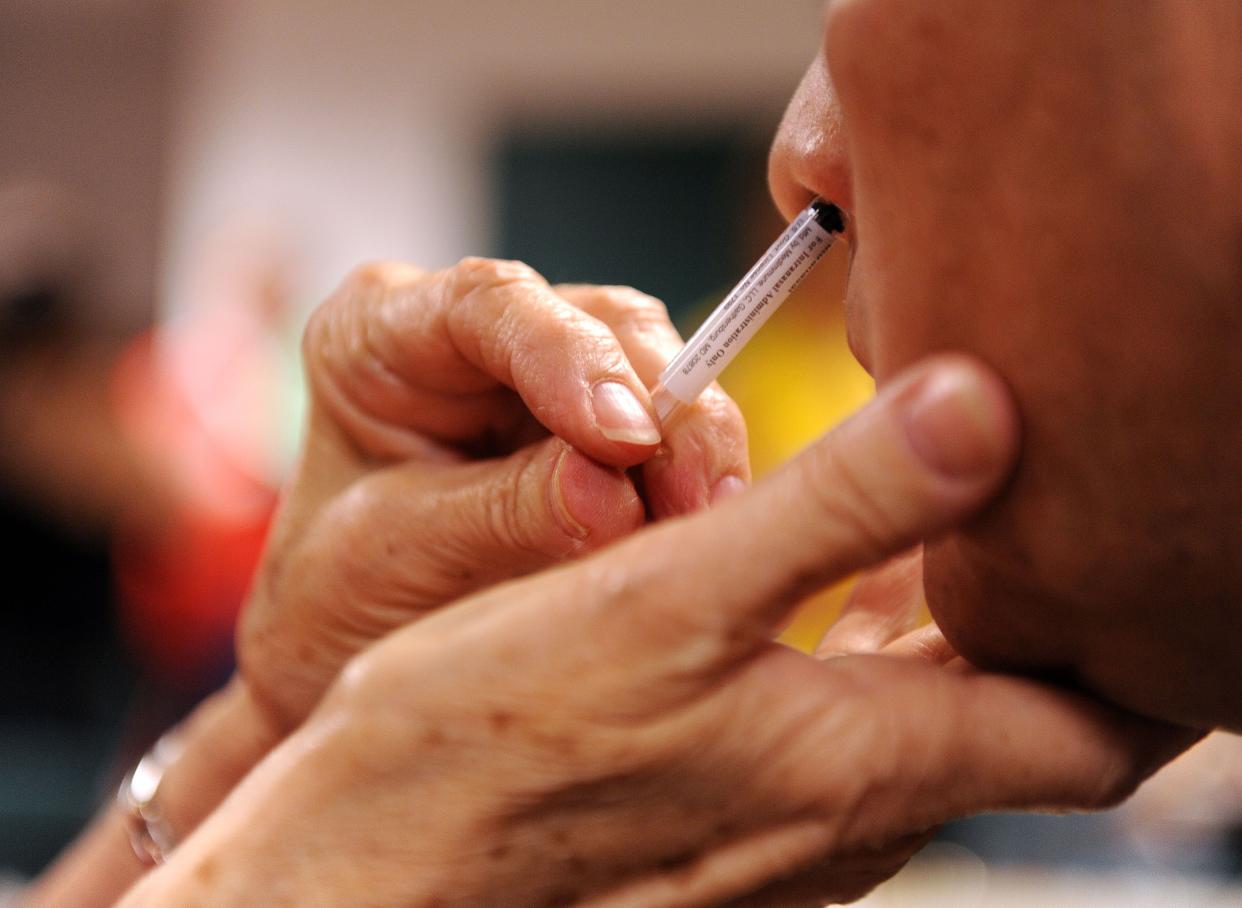
Two doses of the vaccine will be given to the participants, and will be distributed one week apart.
A key component of the vaccine is Protollin, which the hospital describes as an “intranasal agent that stimulates the immune system.”
“This vaccine harnesses a novel arm of the immune system to treat AD,” Tanuja Chitnis, the trial’s principal investigator, said in a statement.
“Research in this area has paved the way for us to pursue a whole new avenue for potentially treating not only AD, but also other neurodegenerative diseases.”
Weiner hopes the vaccine will one day be used as a “nontoxic treatment” for at-risk people and for patients already diagnosed with the disease, which more than 6 million Americans are battling, according to the Alzheimer’s Association.
