More eye medicine recalled in 13-state outbreak of ‘drug-resistant’ bacteria infections
Eye medicine manufacturer Global Pharma will recall one more product, the FDA has announced, in connection to a bacterial infection outbreak that’s now reached 13 states and is linked to one death.
Here’s the latest on the outbreak of what the U.S. Centers for Disease Control and Prevention is calling “a rare strain of extensively drug-resistant” Pseudomonas aeruginosa bacteria.
READ MORE: Death, blindness, eye drops recall: bacterial infections in Florida, Texas, other states
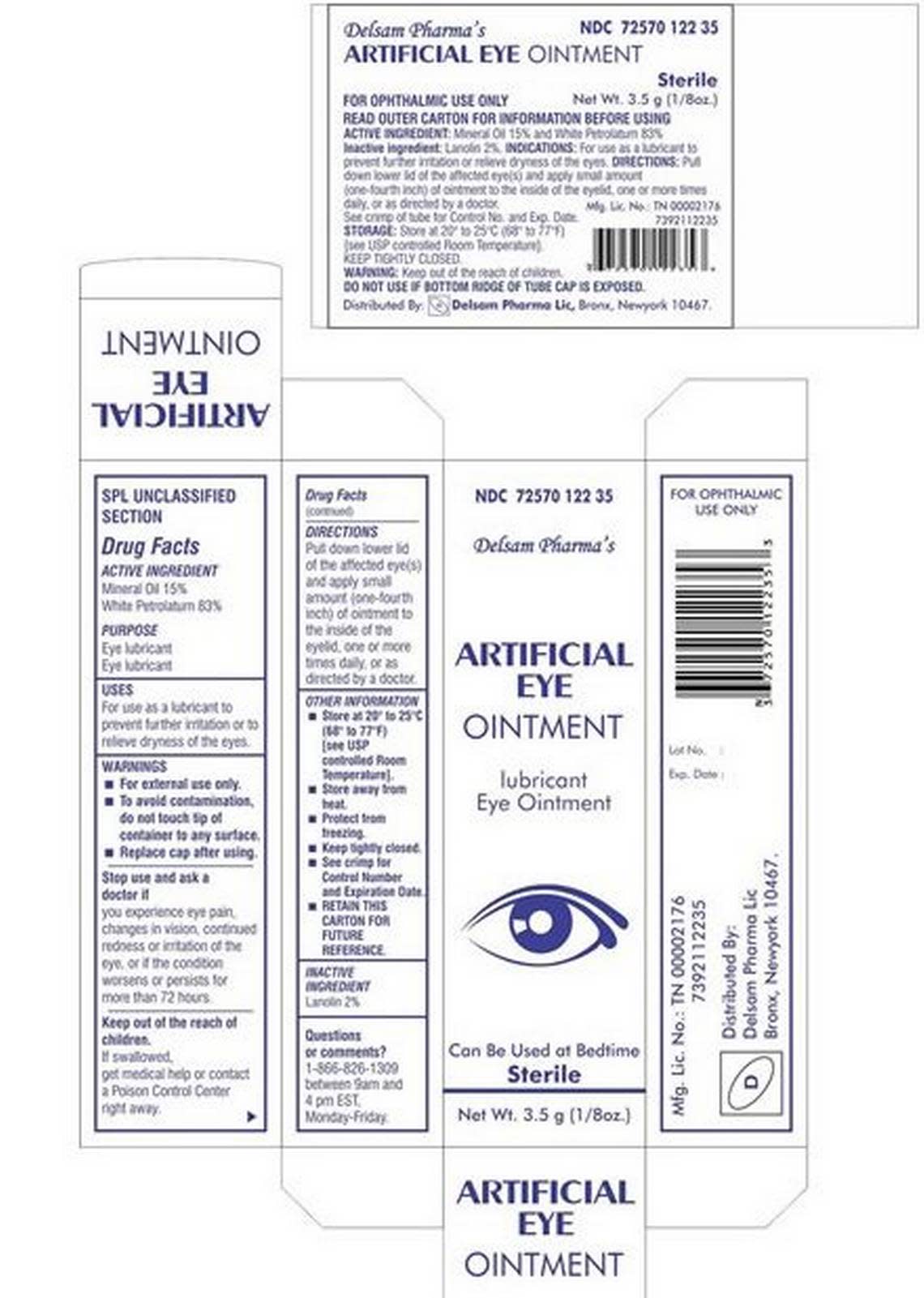
What eye ointment has been recalled?
Information on package: The FDA announced Wednesday, the day after warning consumers and medical professionals not to use Delsam Pharma Artificial Eye Ointment, that Global Pharma agreed to recall the medicine, which carries NDC No. 72570 122 35 on its packaging.
Global Pharma recalled Delsam Pharma’s and EzriCare’s Artificial Tears three weeks ago.
Where has the outbreak reached and how many are ill?
States affected: As of the CDC’s last update, which was Tuesday, 58 people have been sickened in New York, California, Florida, Texas, Colorado, Connecticut, Illinois, New Jersey, New Mexico, Nevada, Utah, Washington and Wisconsin. There’s been one death and five people have suffered vision loss.
Of those 58 people, 35 were in four healthcare facilities.
“Most patients reported using artificial tears,” the CDC said. “Patients reported over 10 different brands of artificial tears and some patients used multiple brands. EzriCare Artificial Tears, a preservative-free, over-the-counter product packaged in multidose bottles, was the brand most commonly reported. This was the only common artificial tears product identified across the four healthcare facility clusters.”
CDC says testing found the strain of Pseudomonas aeruginosa in “multiple lots.” The strain found in open bottles matched the outbreak strain.
What should you do now?
Consumer information: Return the eye ointment to the place of purchase for a full refund.
If you have questions about the Delsam recall, call Delsam at 866-826-1309 or e-mail delsampharma@yahoo.com, Monday through Friday, 11 a.m. to 4 p.m., Eastern time.
If you’ve had any medical problems from this or any other drug, first see a medical professional. Then, let the FDA know via its MedWatch Adverse Event page or by filling out a form you can get by calling 800-332-1088.
