Monroe County pharmaceutical company Baxter is now Simtra, expansion is on horizon

In the cleanest of clean rooms in the center of Simtra’s Bloomington manufacturing facility, high-tech machines fill syringes with medications to treat chronic conditions such as diabetes and life-threatening illnesses such as cancer.
Positive pressure in the room helps keep out contaminants. Workers can enter only after they take off their street clothes, put on hospital-like scrubs and follow strict head-to-toe sterilization protocols. Even the sequence of putting on their clothes is prescribed.
Site Director Patrick J. Adams said on a recent facility tour that the meticulous attention to detail sharply reduces the number of syringes and vials that are discarded. The slightest contamination in a syringe’s contents can render the medication useless.
New jobs: At former Otis Elevator plant, Phoenix Closures plans $20M expansion
Simtra’s Bloomington operation includes the 600,000 square-foot manufacturing site at 927 S. Curry Pike, two warehouses on Curry Pike and a 120,000-square-foot inspection/packaging/labeling facility on Daniels Way, just south of the Cook headquarters.
Some of the lines in Simtra’s Bloomington facility involve floor-to-ceiling glass-and-metal boxes with openings for long gloves into which workers stick their arms to manipulate machinery.

Baxter sells Bloomington facility to investment firms
Simtra BioPharma Solutions formerly was Baxter International’s BioPharma Solutions business. Baxter sold the unit in October for $4.25 billion to investment firms Advent International and Warburg Pincus. Combined, Advent and Warburg Pincus have more than $162 billion in assets under management, according to their websites.
The firms said in a news release that Simtra will be headquartered in New Jersey and will continue its manufacturing operations in Halle, Germany, and Bloomington.
According to filings by Baxter, the unit was projected to generate revenues this year of about $600 million.
Possible expansion for Simtra in Bloomington
Adams, who has been with Baxter/Simtra for years, said the ownership change has created an “energy at the site that’s pretty exciting.”

That’s in part because the new owners are planning big expansions. The site has about 1,000 employees, but Adams said the company plans to construct a new building on its Bloomington campus to add more manufacturing lines in the next two to three years, which would require hiring “hundreds” of additional employees.
The Bloomington site already runs around the clock in three shifts and produces about 130 million products annually, of which about 100 million are syringes, Adams said. Customers stretch the globe — 120 countries — and include major pharmaceutical firms. The Bloomington site produced Moderna’s COVID-19 vaccine, for example, though it does not anymore. Other products include anything from seasonal vaccines to medications for acute or chronic conditions.
The National Institutes of Health project the number of Americans aged 50 and older with at least one chronic condition will double between 2020 and 2050.
Adams said the industry is growing at just over 10% annually, and Simtra hopes to exceed that growth because of its advanced capabilities, which include depyrogenation — using high heat to render objects sterile — and lyophilization, a process that turns liquids into solids, primarily to increase a product’s shelf life and ease of storage by eliminating the need for refrigeration.
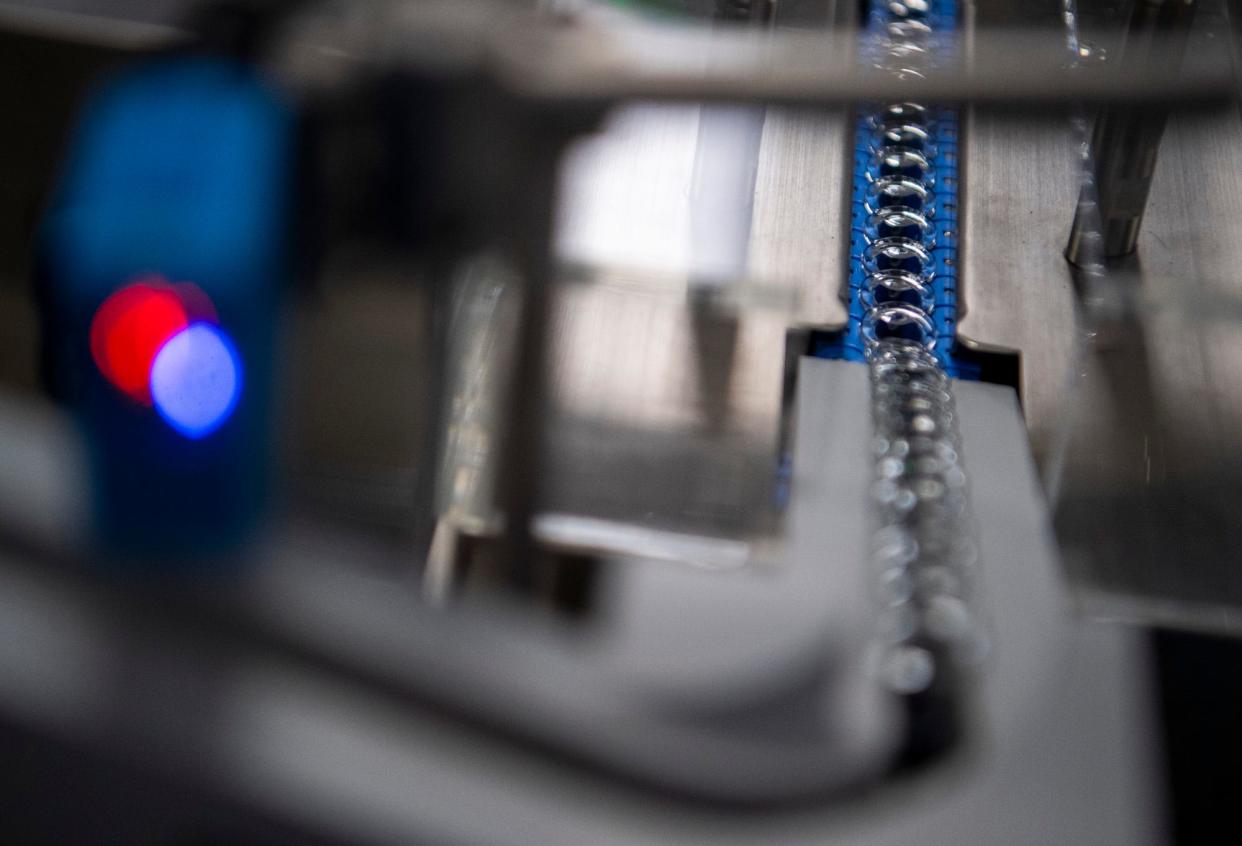
'Ultra clean' labs, multiple inspections for Simtra's Bloomington products
To get into the manufacturing facility’s interior, workers have to undergo months of training to learn how not to compromise the “ultra clean” laboratory. A cubic foot of normal air contains hundreds of thousands of fine particles, while the Simtra facility’s “ultra clean” labs contain no more than 100 particulates, thanks to measures including HEPA filters and positive pressure, Adams said.
Despite all the precautions, every single item produced by Simtra in Bloomington gets inspected multiple times before being cleared for shipping. In the company’s inspection/packaging/labeling facility on Daniels Way, machines turn syringes upside down to shake up their contents before a camera scans them to detect tiny foreign particles. If a syringe passes that inspection, conveyors move it to another “turret,” where cameras inspect the syringe itself and its stopper.
On a recent Tuesday, one of the lines had inspected nearly 200,000 syringes that day, with a rejection rate of 0.89%. Simtra’s goal is less than 2%. A monitor lists reasons for the rejections. If operators spot a trend, Adams said they try to figure out what caused the rising rejection rate — faulty equipment, human error, subpar supplies — to fix the problem.
Inspection is a key part of the process, Adams said, because it “prevents escape to the market.”

The facility undergoes regular inspections by government agencies and customers, who typically visit over several days a year to walk the floor and ask questions. Sometimes, Adams said, they make suggestions for improvement. At other times, they get ideas from the way that Simtra operates.
He said the customers and Simtra work together to reach a common goal: reducing the risk of contamination.
Is Simtra in Bloomington hiring now?
Most of the company’s Bloomington facilities are at or near capacity, but Jennifer Kelley, director of human resources, said the company is hiring for roles including supply chain, maintenance, packaging and production. Applicants need a high school diploma or GED. Experience in related manufacturing is helpful but not required, Kelley said.
Wages in production start near $19 per hour. Kelley said people can apply through the company’s website: tinyurl.com/yfmru69h.
Boris Ladwig can be reached at bladwig@heraldt.com.
This article originally appeared on The Herald-Times: Bloomington pharma company Simtra plans to add hundreds of jobs
