Melatonin makers urged to adopt new guidelines as ER visits rise
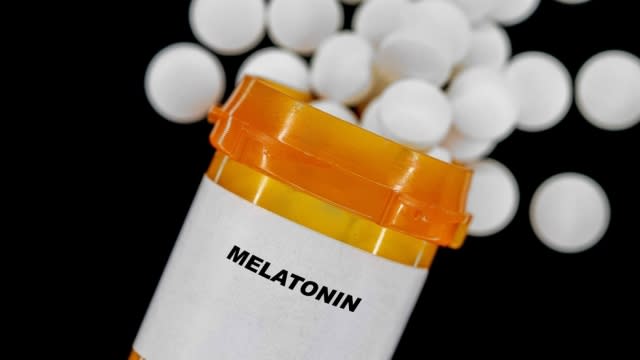
As more children go to the emergency room for taking melatonin without supervision, the Council for Responsible Nutrition has adopted new voluntary packaging guidelines for makers of the hormone supplement to promote its "responsible use." Melatonin supplements are commonly used as a sleep aid, and although generally have few side effects, they can cause issues for those taking high doses.
The Council for Responsible Nutrition adopted guidelines for regular melatonin supplements in addition to the gummy forms.
The new guidelines for melatonin supplements include labels that remind users that melatonin can cause drowsiness, and the implementation of child-deterrent packaging.
New guidelines for gummies were also issued, including a request that manufacturers consider packaging gummy products in containers with child-deterrent closures and labeling advisories that encourage the use of products under appropriate conditions and guidance.
“These are just the latest in a series of voluntary guidelines that CRN members have adopted that underscore CRN’s unwavering commitment to the well-being of consumers and the integrity of the dietary supplement market,” said Council for Responsible Nutrition President Steve Mister. “By setting these high standards, we help our members offer products that are responsibly manufactured and marketed, and widely trusted by consumers.”
SEE MORE: Is melatonin safe for kids?
The new guidelines come over a month after the Centers for Disease Control and Prevention reported that unsupervised exposures to melatonin supplements among young children have substantially increased.
The CDC said there were 11,000 reported emergency department visits for unsupervised melatonin ingestions by young children from 2019–2022.
"Melatonin products do not require child-resistant packaging, although such packaging can be voluntarily implemented," the CDC said. Among ED visits with documentation of container type, approximately three-quarters involved melatonin accessed from bottles, suggesting that infants and children opened bottles or that bottles were not properly closed. Selecting products with child-resistant packaging might be advisable in homes with young children."
The Food and Drug Administration does not regulate supplements like melatonin but notes it can be a relatively low-risk treatment for insomnia.
Taking higher doses can cause drowsiness, daytime sleepiness, headaches and nausea, officials said.
