FDA is warning consumers not to use certain pregnancy tests
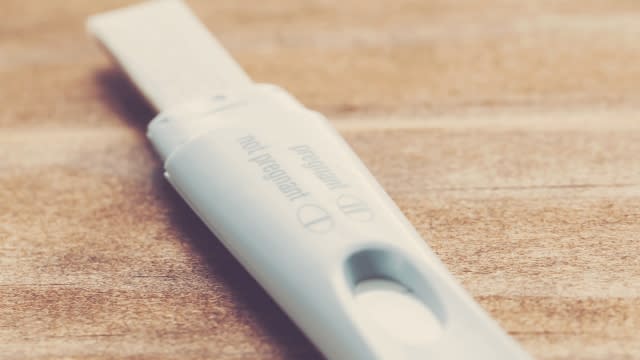
The Food and Drug Administration is asking consumers not to use certain pregnancy, ovulation and urinalysis tests.
The tests were manufactured by Universal Meditech, Inc., which notified the FDA that it had stopped all operations and is no longer providing support for the tests.
"The FDA is not able to confirm the performance of UMI’s tests, raising concerns that the tests may not be safe and effective," the FDA said in a statement.
UMI initiated a recall to stop tests from going to distributors, but it did not recall items already distributed to consumers. That's what prompted an FDA Safety Communication, so customers would know to stop using the tests.
SEE MORE: FDA approves first postpartum depression pill
If you have any of the following products, the FDA says you should throw them out:
One Step Pregnancy Test
DiagnosUS One Step Ovulation Test
HealthyWiser UriTest 10 Parameter Reagent Test Strips for Urinalysis
HealthyWiser UriTest UTI Test Strips
HealthyWiser KetoFast Ketone Test Strips
HealthyWiser pH-Aware pH Test Strips
To Life hCG Pregnancy Urine Test
Am I Pregnant Pregnancy Midstream Test
DeTec hCG Pregnancy Urine Test
PrestiBio Pregnancy Strips
PrestiBio Rapid Detection Pregnancy Test Midstream
PrestiBio Ovulation Strips
PrestiBio Urinalysis Test Strip 10 Parameters
PrestiBio Ketone Test Strips
PrestiBio Breast Milk Alcohol Test Strips
The FDA recommends that people see their medical provider to confirm results if they've recently used one of the tests.
SEE MORE: RFK Jr. backtracks on support for federal abortion ban
