FDA panel recommends updating Covid boosters for the fall
Advisers to the Food and Drug Administration on Thursday recommended updating the Covid vaccines to target a circulating strain of the virus, while pushing for newer vaccines that provide longer-lasting protection.
The FDA’s Vaccines and Related Biological Products Advisory Committee voted unanimously in support of tweaking the shots to target an XBB strain, as well as dropping the original coronavirus strain from the formulation.
The committee did not, however, make a formal recommendation on which specific XBB lineage the updated boosters should target, nor did it make a recommendation on who should get the shots and when. The latter will likely be left up to the Centers for Disease Control and Prevention, which has its own advisory committee meeting next week.
The recommendation will now be handed off to the FDA, which is expected to make a final decision soon on which Covid strain to target. Drugmakers need enough time to produce and distribute the new shots, which are expected to be used as early as September as part of a fall booster campaign.
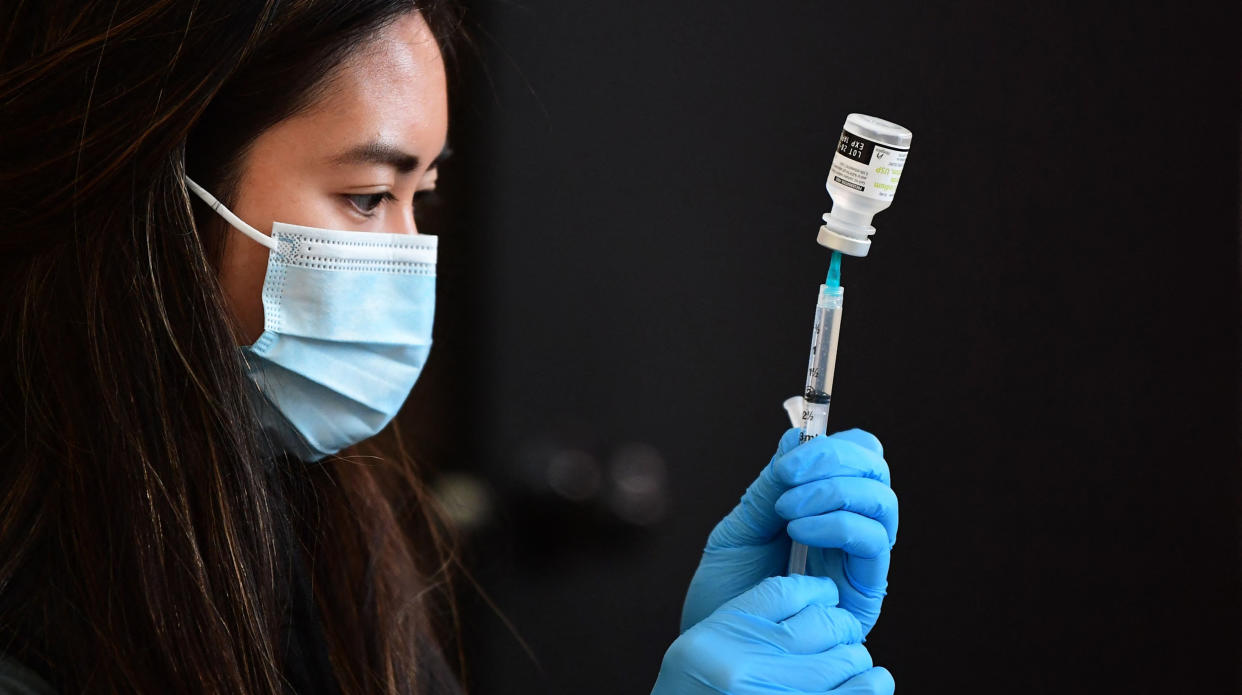
Pfizer said it could distribute reformulated doses as early as the end of July, depending on the strain selected. Moderna said it expects to begin shipping updated doses, pending FDA approval, “by the end of the summer.” Novavax said it could have updated doses available in the fall.
It’s still unclear whether the fall is the best time to offer boosters. Covid’s spread has often been erratic over the past three years, without the clear seasonality seen with viruses like the flu.
“This season will be very telling whether Covid settles into a seasonal pattern or not,” said Ruth Link-Gelles, a senior epidemiologist at the CDC.
During Thursday’s discussion, many committee members appeared to be in support of updating the boosters to target XBB.1.5, a descendent of XBB that began circulating widely last fall and winter and is the predominant strain in the United States. XBB itself is a descendant of two omicron subvariants.
XBB.1.5 "seems to be at the front of the line," said Dr. Peter Marks, the FDA's top vaccine regulator.
XBB.1.5 accounts for about 40% of all new Covid cases as of Saturday, according to the CDC. It’s followed by XBB.1.16 (also called “Arcturus” on social media) and XBB.1.9.1. While the CDC no longer tracks levels of Covid spreading in communities, data from the agency shows hospitalizations have been trending downward since March.
Updating the vaccine with an XBB strain “makes the most sense,” said committee member Dr. Mark Sawyer, an infectious disease specialist at the University of California San Diego School of Medicine. “I think the data is quite clear.”
The XBB strains aren’t too genetically different from the next, meaning an XBB.1.5 vaccine should work well against the newer strains, committee members said.
According to new data from CDC scientists, the current boosters do provide protection against XBB.1.5, although not as much protection as they do BA.4 and BA.5, two strains that are no longer in circulation in the U.S. (The current boosters were formulated to target these strains, as well as the original coronavirus strain.)
On Thursday, the drugmakers presented data from animal studies that suggest an XBB-containing vaccine would provide more protection against the current circulating strains than the current boosters.
Choosing to target an XBB strain falls in line with a recommendation from the World Health Organization in May, as well as the European Medicines Agency, which recommended using XBB-containing vaccines in June.
This will be the second time the Covid vaccines have been updated in the U.S., following the FDA authorizing the BA.4/BA.5 boosters last year.
In addition to adding an XBB strain, the committee supported dropping the original coronavirus strain from the vaccine, with several members saying they didn’t see an advantage to including it. That strain has been included in the vaccines since they were first authorized back in December 2020, but has long been out of circulation globally.
Jerry Weir, director of the Division of Viral Products in the FDA’s Office of Vaccines Research and Review, said an XBB-only vaccine could improve vaccine effectiveness because the immune system won’t be hampered by its response to the original coronavirus strain — a phenomenon known as immunological imprinting.
“I do think taking out the ancestral strain will do something to optimize the response,” he said.
While updating the boosters was supported by the committee, some members stressed the need for investment to find more effective and longer-lasting vaccines. The existing vaccines are highly effective, particularly against severe illness, but their effectiveness against infection generally wanes after a few months.
“People are tired of the pandemic, people feel like we have vaccines, we’re done,” said committee member Dr. Ofer Levy, the director of the Precision Vaccines Program at Boston Children’s Hospital, “but I think our discussion reflects the challenge ahead and the need for further innovation.”
Uptake of the booster shots has been low to date. Only 17% of the U.S. population has gotten an updated booster, according to the CDC.
Follow NBC HEALTH on Twitter & Facebook.
