Eye drop brands recalled due to bacterial infections in Texas and 11 other states
Two eye drop brands were recalled due to an “extensively drug-resistant” bacteria identified by the Centers for Disease Control and Prevention.
The recall impacted 55 patients across 12 states including Texas, Florida and New York.
What eye drop brands are recalled?
Global Pharma Healthcare voluntarily recalled all expiration dates of artificial tears lubricant eye drops by EzriCare and Delsam Pharma due to possible contamination.
The EzriCare Artificial Tears have NDC No. 79503-0101-15 and UPC code 3 79503 10115 7.
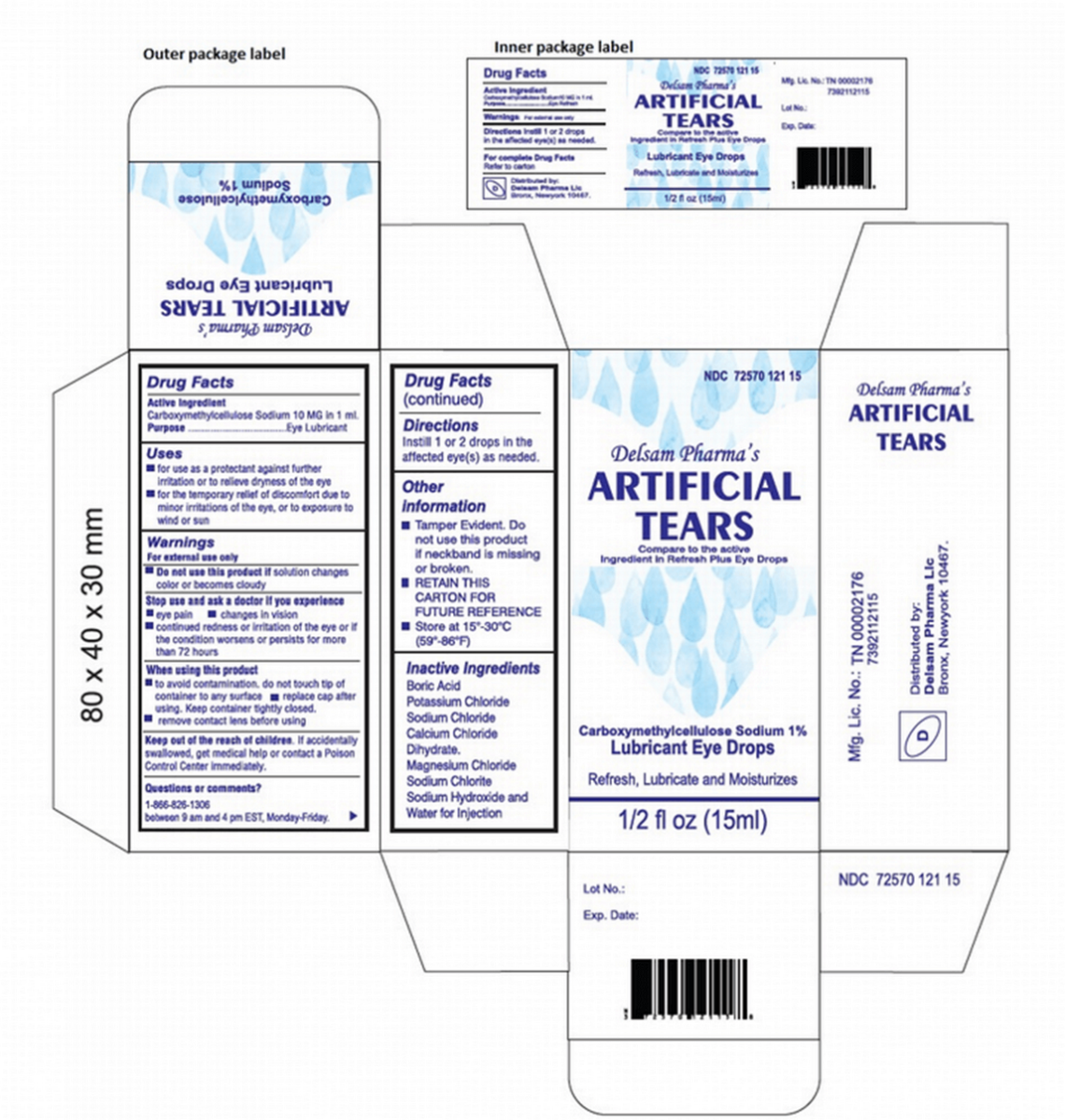
The Delsam Pharma Artificial Tears have NDC No. 72570-121-15 and UPC code 72570-0121-15.
Why were the eye drops recalled?
The U.S. Food & Drug Administration reports use of contaminated artificial tears can result in the risk of eye infections and permanent loss of vision.
Serious effects from the eye drops reported by the 55 patients include eye infections and permanent loss of vision. As of Feb. 2, one death caused by a bloodstream infection was reported.
I have the recalled eye drops, what now?
If you own the EzriCare or Delsam Pharma eye drops, stop using them.
Contact your physician or healthcare provider if you’ve experienced problems from using the eye drops.
Anyone with questions about the recall can contact the distributors:
Ezricare: 1-516-715-5181 or arupharmainc@yahoo.com from Monday to Friday, 11 a.m. to 4 p.m. Eastern time
DELSAM Pharma: 1-866-826-1306 or delsampharma@yahoo.com from Monday to Friday from 11 a.m. to 4 p.m. Eastern time.
