CONFIRMED: The maker of EpiPen overcharged the government, and lawmakers are furious
The Centers for Medicare & Medicaid Services (CMS) confirmed to lawmakers that Mylan, the drug company that makes EpiPen, has been overcharging the government for the life-saving allergy medication.
And naturally, lawmakers are furious about it. Here's a statement from Senator Ron Wyden (D-OR):
"Today's letter is more evidence that while Mylan irresponsibly raised the price of EpiPen, they were also bilking taxpayers out of millions of dollars," Wyden and Pallone said."Essential medicines like EpiPen are increasingly out of reach for families across the nation due to unjustified price hikes, and it's high time for drug companies to take responsibility for their actions. We will ensure taxpayers get their due."
Wyden signed a letter that a number of members of the Senate Finance Committee sent to the CMS Inspector General last month. What the senators wanted to know was whether or not Mylan manipulated the Medicaid Drug Rebate Program (MDRP) by classifying EpiPen as a generic drug when it is in fact not.
The short answer from CMS that we have now is: Yes, but they're not sure by how much yet. Here's part of the letter the agency sent to the Senate [emphasis ours].
A review of our records indicates that, prior to 1997, EpiPen was reported as a single source, or brand drug, for the Medicaid Drug Rebate Program. Since the fourth quarter of 1997, EpiPen has been reported as a non-innovator multiple source, or generic drug. EpiPen is approved under a New Drug Application (NDA) by the Food and Drug Administration (FDA), has patent protection, and has no FDA-approved therapeutic equivalents.
These facts indicate EpiPen does not meet the definition of a multiple source drug, but, in fact, meets the definition of a single source drug or brand drug. The Center for Medicaid and CHIP Services in CMS has, on multiple occasions, provided guidance to the industry and Mylan on the proper classification of drugs and has expressly told Mylan that the product is incorrectly classified.
This incorrect classification has financial consequences for the amount that federal and state governments spend because it reduces the amount of quarterly rebates Mylan owes for EpiPen.
The reason why misclassification is a big deal is because brand-name drugs have inflation protections with Medicaid that generics don't. Brand-name drugs have to pay higher rebates to states than generics — 23.1% versus 13%. Also, brand-name drugs have to pay additional rebates if their price increases rise more than inflation.
So by being classified as a generic Mylan saved itself a bunch of money. Back in 2009, Mylan paid a $124 million fine for misclassifying its drugs and under-paying rebates this way. It is the responsibility of the manufacturer to maintain accurate information of its drug's status.
Not done
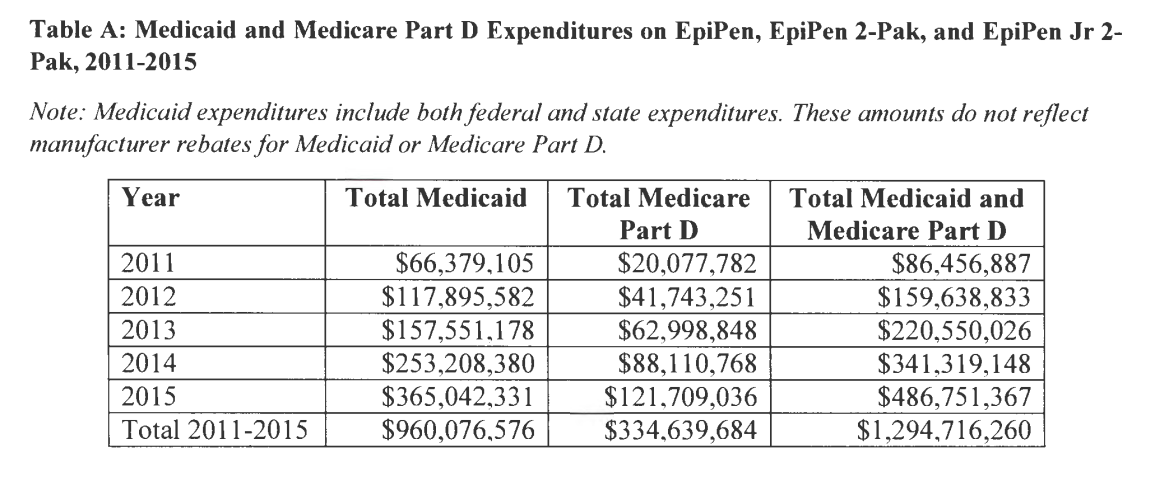
From 2011 to 2015, government spending on EpiPen increased 463%, from $86 million to $487 million, according to CMS. This table was included in their letter.
Reuters
Now it looks like the government may want a chunk of that back.
Mylan came under fire in August when it increased the price of a pack of two EpiPens to $608. The pack cost around $100 when Mylan bought the drug back in 2007. The company's CEO, Heather Bresch, was on Capitol Hill answering for this last month, but so far lawmakers have in no way been mollified.
In the House, Representatives Jason Chaffetz (R-UT) and Elijah Cummings (D-MD) are still demanding more information about how much Mylan actually makes off EpiPens, for one thing. They demanded more information from the company earlier this week.
So just add this to a list of huge problems for the company.
See images of the life-saving medication:
NOW WATCH: Self-made millionaire reveals the biggest money mistake you might be making
More from Business Insider:
