New Alzheimer's Drug Study Seeking More Diverse Volunteers
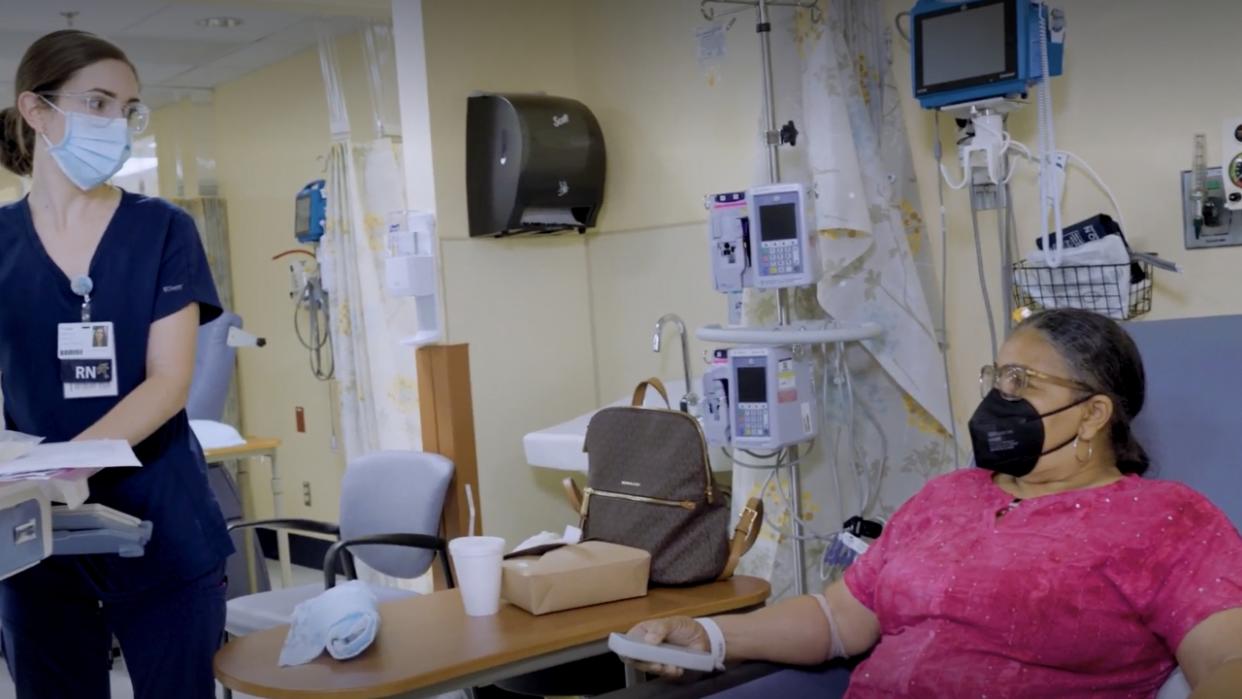
Carol Turner got an IV that may contain the Alzheimer's drug lecanemab at a clinic at Eastern Virginia Medical School. She is in one of the next big clinical trials for the drug, which is called "The AHEAD Study."
The FDA gave accelerated approval to the treatment earlier this month for patients with mild cognitive problems or mild dementia.
But Turner hasn't been diagnosed. She has no symptoms.
"The population that we are right now targeting, are much, much earlier and, probably, 20 years before they develop any symptoms," said Eastern Virginia Medical School Professor Hamid Okhravi.
More than 70 research teams across the country are trying to recruit 1,400 participants age 55 to 80 with pre-clinical or early pre-clinical Alzheimer's for the study.
That's when someone does not show outward signs of the disease, but brain scans can show deposits of amyloid-beta, which is a hallmark of Alzheimer's.
SEE MORE: Study: New Drug Appears To Slow Alzheimer's
"Maybe we need to move a little bit earlier to be able to bend the curve even much, much stronger than before," Okhravi said.
About 1 in 8 people over age 65 have Alzhiemer's. That includes Turner's mom.
She and her brother signed up for the trial because their father died from the disease.
"It was something that we never thought we would hear because, you know, there's nothing out here — definitely not for our age," Turner said. "So we figured that would just be awesome. And as soon as it launched, we jumped on board."
Turner was the first Black participant to volunteer.
She hopes many more Black individuals with family history will sign up, too.
Older Black Americans are twice as likely as older White Americans to have Alzheimer's or other dementia. And research hasn't been able to answer why.
SEE MORE: Lack Of AAPI Participants In Alzheimer's Study Concerns Researchers
On top of that, there is a chronic, uneven number of Black Americans in clinical trials overall- including for new medicines.
A Bloomberg News analysis showed only 2% of patients included in trials reported in the past decade were Black.
In the previous Phase 3 lecanemab trial, the number of Black participants were dramatically lower than White participants.
What all of that means is that when drug makers are developing an Alzheimer's treatment, they're missing a full picture of how well that medicine works.
"We really need to have participation from different walk of life, and also different races and ethnicities," Okhravi concluded.
The AHEAD Study will last for about 4 years, with results expected in 2028.
